Introducing the Moles Molecules and Grams Worksheet Key, an indispensable resource for navigating the intricacies of chemistry. This guide delves into the fundamental concepts of mole, molecular mass, Avogadro’s number, stoichiometry, limiting reactants, percent yield, and acid-base titrations, providing a comprehensive understanding of these critical topics.
As you embark on this journey, prepare to unravel the mysteries of chemical reactions, quantify substances, and explore the fascinating world of chemistry with newfound clarity and confidence.
Mole Concept
The mole is a unit of measurement for the amount of substance. It is defined as the amount of substance that contains as many elementary entities as there are atoms in 0.012 kilograms of carbon-12. The mole is a very large unit, so it is often convenient to use smaller units, such as the millimole (mmol) or the micromole (µmol).
Moles are used in chemistry to express the amount of reactants and products in a chemical reaction. For example, the balanced chemical equation for the combustion of methane is:
“`CH₄ + 2O₂ → CO₂ + 2H₂O“`
This equation tells us that one mole of methane reacts with two moles of oxygen to produce one mole of carbon dioxide and two moles of water.
Moles can also be used to convert between different units of measurement. For example, we can use the molar mass of a compound to convert between grams and moles. The molar mass of a compound is the mass of one mole of that compound.
For example, the molar mass of methane is 16.04 g/mol. This means that one mole of methane has a mass of 16.04 grams.
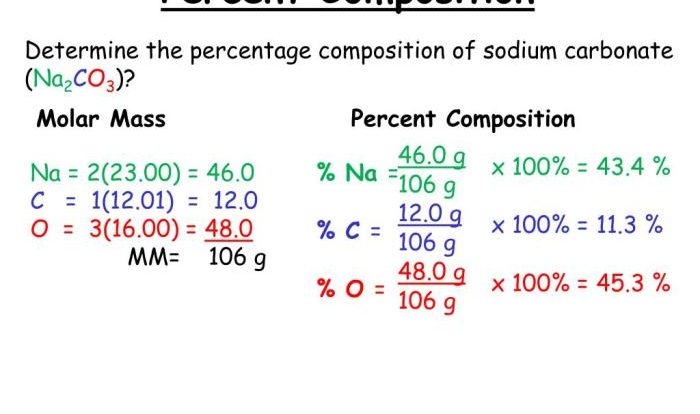
Molecular Mass
Molecular mass is the mass of one molecule of a compound. It is calculated by adding together the atomic masses of all the atoms in the molecule. For example, the molecular mass of methane (CH₄) is:
“`
01 g/mol (C) + 4(1.01 g/mol) (H) = 16.04 g/mol
“`
Molecular mass is an important property of compounds because it can be used to calculate the molar mass of the compound. The molar mass of a compound is the mass of one mole of that compound. It is calculated by multiplying the molecular mass of the compound by the number of moles of the compound.
Avogadro’s Number
Avogadro’s number is the number of atoms in one mole of a substance. It is equal to 6.022 × 10^23 atoms/mol. Avogadro’s number is a very large number, so it is often convenient to use smaller units, such as the millimole (mmol) or the micromole (µmol).
Avogadro’s number can be used to convert between moles and molecules. For example, we can use Avogadro’s number to convert between the number of molecules of methane in one mole of methane and the number of moles of methane in one molecule of methane:
“`
- mol CH₄ = 6.022 × 10^23 molecules CH₄
- molecule CH₄ = 1.66 × 10^-24 mol CH₄
“`
Stoichiometry, Moles molecules and grams worksheet key
Stoichiometry is the study of the quantitative relationships between reactants and products in chemical reactions. Stoichiometry can be used to balance chemical equations, which are equations that show the reactants and products of a chemical reaction in their correct proportions.
For example, the balanced chemical equation for the combustion of methane is:
“`CH₄ + 2O₂ → CO₂ + 2H₂O“`
This equation tells us that one mole of methane reacts with two moles of oxygen to produce one mole of carbon dioxide and two moles of water.
Stoichiometry can also be used to calculate the amount of reactants or products that are needed or produced in a chemical reaction. For example, we can use stoichiometry to calculate the amount of oxygen that is needed to burn one mole of methane:
“`
mol CH₄ + 2 mol O₂ → 1 mol CO₂ + 2 mol H₂O
“`
This equation tells us that one mole of methane reacts with two moles of oxygen. Therefore, we need two moles of oxygen to burn one mole of methane.
Frequently Asked Questions: Moles Molecules And Grams Worksheet Key
What is the significance of Avogadro’s number in chemistry?
Avogadro’s number, approximately 6.022 x 10^23, serves as a fundamental constant in chemistry, representing the number of entities (atoms, molecules, ions) present in one mole of a substance. It enables the conversion between the mass and number of particles, facilitating precise calculations and quantitative analysis.
How does stoichiometry help balance chemical equations?
Stoichiometry plays a crucial role in balancing chemical equations by ensuring that the number of atoms of each element on the reactants’ side matches the number of atoms of the same element on the products’ side. This adherence to the law of conservation of mass guarantees accurate representation of chemical reactions and enables the prediction of product quantities.
What factors can affect the percent yield of a chemical reaction?
Several factors can influence the percent yield of a chemical reaction, including the purity of the reactants, side reactions, incomplete reactions, and losses during the reaction process. Additionally, reaction conditions such as temperature, pressure, and the presence of a catalyst can impact the efficiency of the reaction and ultimately affect the percent yield.