Molecular mass and percent composition worksheet answer key – Welcome to our comprehensive guide on molecular mass and percent composition, accompanied by a detailed worksheet answer key. This guide will delve into the fundamental concepts, formulas, and step-by-step calculations for determining molecular mass and percent composition, empowering you with a solid understanding of these essential chemistry principles.
Our engaging narrative unravels the complexities of molecular mass and percent composition, making them accessible and understandable. Whether you’re a student seeking clarity or an educator searching for valuable resources, this guide has something to offer.
Molecular Mass and Percent Composition
Molecular mass and percent composition are fundamental concepts in chemistry that provide valuable information about the composition and structure of compounds.
Molecular Mass
Molecular mass, also known as molar mass, is the mass of one molecule of a compound expressed in atomic mass units (amu). It is calculated by adding the atomic masses of all the atoms in the molecule.
Formula for calculating molecular mass:
Molecular mass = (Number of atoms of each element) × (Atomic mass of each element)
Example:
- Water (H 2O): (2 × 1 amu) + (1 × 16 amu) = 18 amu
- Sodium chloride (NaCl): (1 × 23 amu) + (1 × 35.5 amu) = 58.5 amu
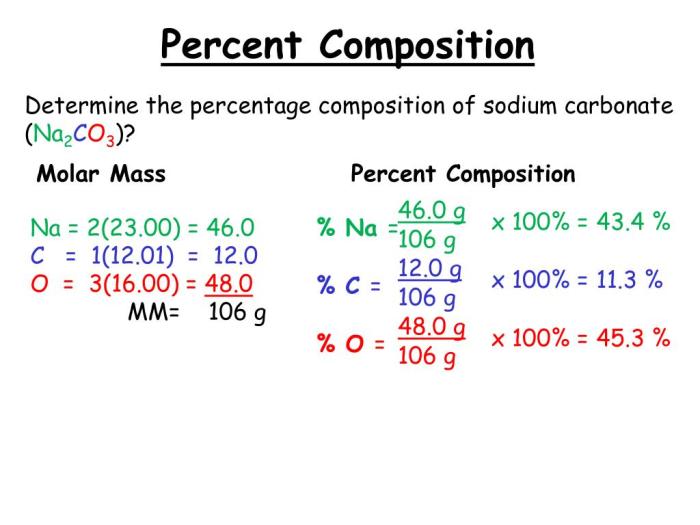
Percent Composition
Percent composition refers to the percentage by mass of each element in a compound. It is calculated by dividing the mass of each element by the total mass of the compound and multiplying by 100.
Steps for calculating percent composition:
- Determine the molecular mass of the compound.
- Find the mass of each element in the compound by multiplying its atomic mass by the number of atoms of that element.
- Calculate the percent composition by dividing the mass of each element by the molecular mass and multiplying by 100.
Example:
Calculate the percent composition of water (H 2O):
- Molecular mass: 18 amu
- Mass of hydrogen: (2 × 1 amu) = 2 amu
- Mass of oxygen: (1 × 16 amu) = 16 amu
- Percent composition of hydrogen: (2 amu / 18 amu) × 100 = 11.11%
- Percent composition of oxygen: (16 amu / 18 amu) × 100 = 88.89%
Worksheet, Molecular mass and percent composition worksheet answer key
Practice Problems:
- Calculate the molecular mass of glucose (C 6H 12O 6).
- Find the percent composition of sodium chloride (NaCl).
Answer Key:
- Molecular mass of glucose: 180 amu
- Percent composition of sodium chloride:
- Sodium: 39.34%
- Chlorine: 60.66%
Questions and Answers: Molecular Mass And Percent Composition Worksheet Answer Key
What is molecular mass?
Molecular mass is the sum of the atomic masses of all the atoms in a molecule.
How do I calculate molecular mass?
To calculate molecular mass, multiply the atomic mass of each element by the number of atoms of that element in the molecule, then add the products together.
What is percent composition?
Percent composition is the percentage by mass of each element in a compound.
How do I calculate percent composition?
To calculate percent composition, divide the mass of each element in the compound by the total mass of the compound, then multiply by 100%.