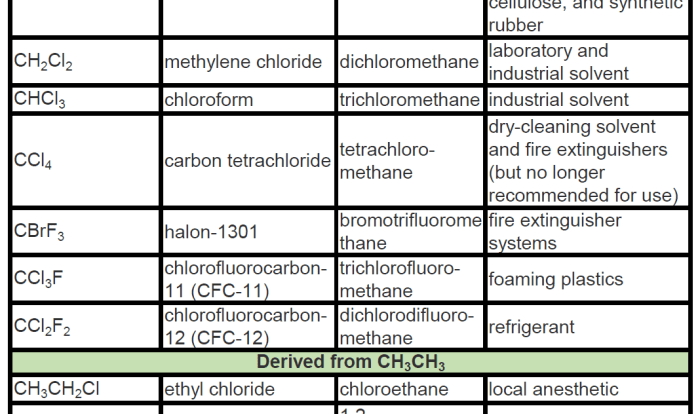
Draw the enantiomer of the compound below – Delving into the fascinating realm of enantiomers, this comprehensive guide will equip you with the knowledge and skills to effortlessly draw the enantiomer of any given compound. From understanding the fundamental concepts of chirality and optical isomers to mastering the practical steps involved in enantiomer drawing, this guide will empower you to navigate the intricacies of enantiomeric chemistry with confidence.
Throughout this exploration, we will delve into the physical and chemical properties of enantiomers, unravel their interactions with chiral molecules, and uncover their diverse applications across various scientific disciplines. By the end of this journey, you will possess a profound understanding of enantiomers, their significance, and their impact on our world.
Define Enantiomers
Enantiomers are stereoisomers that are mirror images of each other and cannot be superimposed. They have the same molecular formula and connectivity but differ in the spatial arrangement of their atoms.
Chirality is the property of a molecule that makes it non-superimposable on its mirror image. A chiral molecule has a chiral center, which is an atom that is bonded to four different groups.
Optical isomers are stereoisomers that have the same molecular formula and connectivity but differ in their ability to rotate plane-polarized light. Enantiomers are a type of optical isomer.
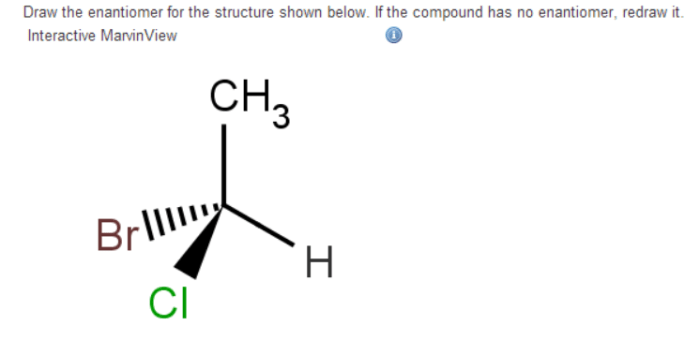
Examples of Enantiomers, Draw the enantiomer of the compound below
- Lactic acid (CH 3CH(OH)COOH)
- Alanine (CH 3CH(NH 2)COOH)
- Ibuprofen (C 13H 18O 2)
Drawing Enantiomers
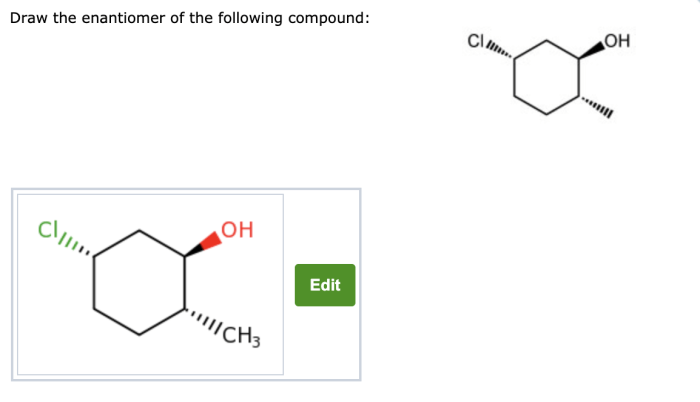
Identifying the Chiral Center
To identify the chiral center in a compound, look for an atom that is bonded to four different groups.
Steps Involved in Drawing the Enantiomer
- Identify the chiral center.
- Draw the mirror image of the molecule.
- Invert the configuration of the groups around the chiral center.
Properties of Enantiomers
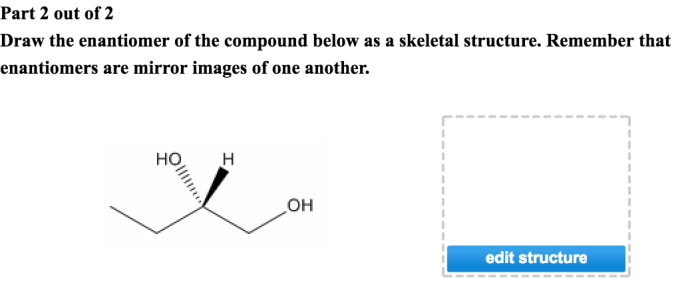
Enantiomers have the same physical and chemical properties, except for their interaction with chiral molecules.
Enantiomers interact with chiral molecules in a different way. This is because chiral molecules have a specific shape that can only fit one enantiomer.
Applications of Enantiomers
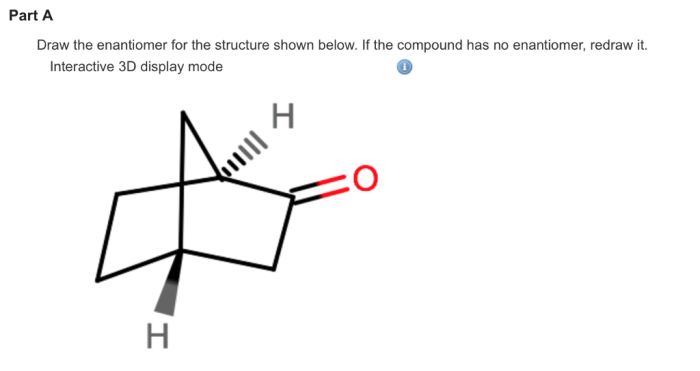
Enantiomers are used in a variety of applications, including:
- Drug development
- Food additives
- Cosmetics
- Pesticides
Importance of Enantiomeric Purity in Drug Development
Enantiomeric purity is important in drug development because enantiomers can have different biological activities. For example, one enantiomer of a drug may be effective, while the other enantiomer may be inactive or even harmful.
Advanced Topics
Use of Spectroscopy and Chromatography in Enantiomer Analysis
Spectroscopy and chromatography are two techniques that can be used to analyze enantiomers.
Spectroscopy can be used to identify the different functional groups in a molecule. This information can then be used to determine the stereochemistry of the molecule.
Chromatography can be used to separate enantiomers. This information can then be used to determine the enantiomeric purity of a sample.
Diastereomers and Their Relationship to Enantiomers
Diastereomers are stereoisomers that are not mirror images of each other. They have the same molecular formula and connectivity but differ in the spatial arrangement of their atoms.
Enantiomers are a type of diastereomer.
FAQ Section: Draw The Enantiomer Of The Compound Below
What is the difference between an enantiomer and a diastereomer?
Enantiomers are non-superimposable mirror images of each other, while diastereomers are non-superimposable stereoisomers that are not mirror images.
How can I determine if a compound is chiral?
A compound is chiral if it contains a chiral center, which is a carbon atom bonded to four different groups.
What are the applications of enantiomers?
Enantiomers have numerous applications in pharmaceuticals, agrochemicals, and fragrances, among other fields.