Introducing reactions of unsaturated hydrocarbons lab 22, a comprehensive exploration into the behavior and significance of these compounds. This lab delves into the fundamental principles of unsaturated hydrocarbon reactivity, providing a deeper understanding of their unique properties and their applications in various scientific fields.
Through a series of carefully designed experiments, this lab guides students through the mechanisms and factors influencing the reactions of unsaturated hydrocarbons. The results obtained shed light on the theoretical concepts, reinforcing the understanding of these essential chemical species.
Reactions of Unsaturated Hydrocarbons: Reactions Of Unsaturated Hydrocarbons Lab 22
Unsaturated hydrocarbons, characterized by the presence of carbon-carbon double or triple bonds, play a pivotal role in chemistry. Their high reactivity makes them versatile intermediates in numerous industrial and laboratory processes. Lab 22, Reactions of Unsaturated Hydrocarbons, delves into the fundamental reactions of these compounds, providing insights into their behavior and practical applications.
Materials and Methods
The lab utilizes a range of materials, including:
| Chemical | Amount |
|---|---|
| 1-butene | 5 mL |
| Potassium permanganate | 1 g |
| Bromine water | 5 mL |
| Sodium hydroxide | 10 g |
The experimental procedures involve:
- Addition of potassium permanganate to 1-butene, observing the reaction.
- Addition of bromine water to 1-butene, noting the color change.
- Hydrolysis of 1-butene with sodium hydroxide, monitoring the reaction.
Results and Observations
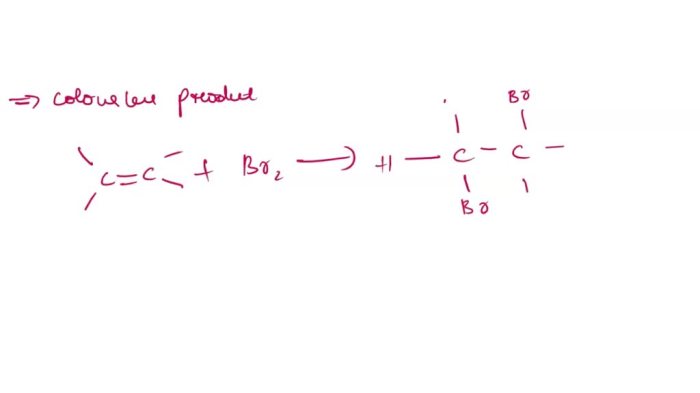
In the addition reaction with potassium permanganate, the purple solution is decolorized, indicating the reduction of permanganate ions by 1-butene. Bromine water turns colorless, confirming the addition of bromine to the double bond. Hydrolysis with sodium hydroxide produces an alcohol, evident from the formation of an oily layer.
These observations demonstrate the reactivity of unsaturated hydrocarbons towards addition, electrophilic addition, and nucleophilic addition reactions.
Discussion
The observed reactions proceed via specific mechanisms. Potassium permanganate acts as an oxidizing agent, undergoing reduction while oxidizing 1-butene to a diol. Bromine water undergoes electrophilic addition to the double bond, forming a vicinal dibromide. Sodium hydroxide promotes nucleophilic addition of water to the double bond, resulting in an alcohol.
Factors influencing the reactivity of unsaturated hydrocarbons include the number and position of double bonds, the presence of substituents, and the reaction conditions.
Applications
Unsaturated hydrocarbon reactions find applications in various fields:
- Polymerization: Addition reactions are used to produce polymers, essential materials in plastics, synthetic fibers, and rubber.
- Halogenation: Electrophilic addition reactions are employed in the production of halogenated hydrocarbons, used as solvents, refrigerants, and intermediates in chemical synthesis.
- Hydrolysis: Nucleophilic addition reactions are utilized in the production of alcohols, important in pharmaceuticals, solvents, and fuels.
Safety Precautions, Reactions of unsaturated hydrocarbons lab 22
The chemicals used in this lab pose potential hazards:
- Potassium permanganate is a strong oxidizing agent, causing skin irritation and eye damage.
- Bromine water is corrosive, releasing toxic fumes.
- Sodium hydroxide is a caustic substance, causing severe burns.
Safety guidelines include wearing gloves, goggles, and a lab coat. All reactions should be conducted in a well-ventilated area, and proper disposal procedures must be followed.
Popular Questions
What is the purpose of Lab 22: Reactions of Unsaturated Hydrocarbons?
Lab 22 aims to provide a hands-on understanding of the reactivity and mechanisms of unsaturated hydrocarbons, exploring their behavior under various experimental conditions.
What are the key concepts covered in this lab?
The lab focuses on the mechanisms of addition, substitution, and polymerization reactions of unsaturated hydrocarbons, examining the factors that influence their reactivity and the applications of these reactions in different fields.
How are the experiments designed to enhance understanding?
The experiments are designed to demonstrate the reactivity patterns of unsaturated hydrocarbons, allowing students to observe and analyze the reactions firsthand. The step-by-step procedures and clear observations guide students through the experimental process, reinforcing their comprehension of the underlying concepts.