Embark on a captivating journey with the Chemistry Mole Packet Answer Key, your ultimate guide to mastering mole calculations, stoichiometry, and chemical reactions. Prepare to delve into a world where numbers dance and equations ignite your understanding of chemistry.
From the intricacies of mole conversions to the precision of stoichiometric ratios, this answer key unlocks the secrets of chemical interactions, empowering you to navigate the complexities of chemistry with confidence.
Chemistry Mole Packet
The Chemistry Mole Packet is a comprehensive resource designed to provide students with a thorough understanding of the mole concept and its applications in chemical calculations.
The packet covers key concepts such as the definition of the mole, Avogadro’s number, molar mass, and stoichiometry. It includes practice problems and examples to reinforce understanding and develop problem-solving skills.
Avogadro’s Number
Avogadro’s number is a fundamental constant in chemistry, representing the number of atoms, molecules, or ions present in one mole of a substance. It is equal to 6.022 x 10^23.
Avogadro’s number is used to convert between the number of particles and the number of moles of a substance. For example, if we know the number of atoms in a sample, we can use Avogadro’s number to determine the number of moles of that substance present.
Molar Mass
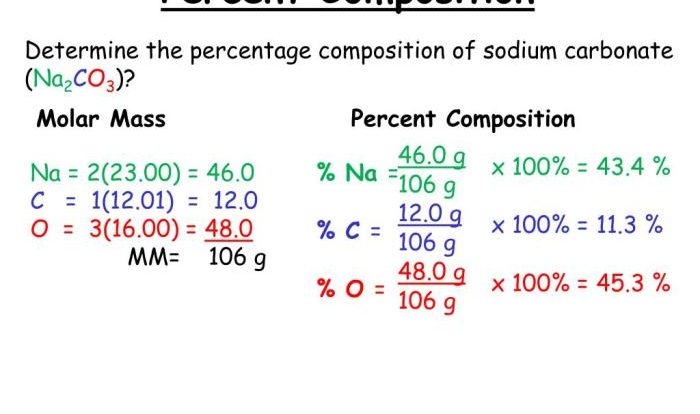
The molar mass of a substance is the mass of one mole of that substance. It is expressed in grams per mole (g/mol).
The molar mass of a substance can be used to convert between the mass and the number of moles of a substance. For example, if we know the mass of a sample, we can use the molar mass to determine the number of moles of that substance present.
Stoichiometry
Stoichiometry is the study of the quantitative relationships between reactants and products in chemical reactions.
Stoichiometry can be used to predict the amount of reactants and products involved in a chemical reaction. For example, if we know the stoichiometry of a reaction, we can calculate the amount of reactants needed to produce a certain amount of product.
Mole Calculations: Chemistry Mole Packet Answer Key
In chemistry, a mole is a fundamental unit used to measure the amount of a substance. It is defined as the amount of substance that contains exactly 6.022 × 10 23elementary entities, which can be atoms, molecules, ions, or electrons.
Mole calculations are essential for determining the number of moles in a given mass of a substance, as well as for converting between different units of measurement. To perform mole calculations, we need to understand the concept of molar mass, which is the mass of one mole of a substance.
Calculating the Number of Moles
To calculate the number of moles in a given mass of a substance, we can use the following formula:
Number of moles = Mass of substance (g) / Molar mass (g/mol)
For example, if we have 100 g of sodium chloride (NaCl), the molar mass of which is 58.44 g/mol, we can calculate the number of moles as follows:
Number of moles = 100 g / 58.44 g/mol = 1.71 moles
Significance of Molar Mass
Molar mass is a significant concept in mole calculations because it provides a direct relationship between the mass and the number of moles of a substance. By knowing the molar mass of a substance, we can easily convert between mass and moles, which is essential for various chemical calculations.
Examples of Mole Calculations
Here are a few examples of mole calculations involving various substances:
- Example 1:Calculate the number of moles in 25 g of glucose (C 6H 12O 6), which has a molar mass of 180.16 g/mol.
- Example 2:Calculate the mass of 0.5 moles of sodium hydroxide (NaOH), which has a molar mass of 40.00 g/mol.
- Example 3:Convert 10.0 g of calcium carbonate (CaCO 3) to moles.
Number of moles = 25 g / 180.16 g/mol = 0.139 moles
Mass of substance = Number of moles × Molar mass
Mass of substance = 0.5 moles × 40.00 g/mol = 20.00 g
Molar mass of CaCO 3= 100.09 g/mol
Number of moles = 10.0 g / 100.09 g/mol = 0.100 moles
Stoichiometry and Mole Ratios
Stoichiometry is the study of the quantitative relationships between reactants and products in chemical reactions. It helps us predict the amounts of reactants and products involved in a given reaction.
Mole ratios are used to determine the quantities of reactants and products in a reaction. A mole ratio is a ratio of the number of moles of one substance to the number of moles of another substance in a chemical reaction.
Mole ratios are derived from the coefficients in a balanced chemical equation.
Using Mole Ratios
To use mole ratios, follow these steps:
- Balance the chemical equation.
- Convert the given amount of one substance to moles.
- Use the mole ratio to determine the moles of the other substance.
- Convert the moles of the other substance to the desired unit (e.g., grams, liters).
Example
Consider the reaction:
2 H 2+ O 2→ 2 H 2O
If we start with 4 moles of hydrogen (H 2), how many moles of water (H 2O) will be produced?
- The balanced equation shows that 2 moles of H2react with 1 mole of O 2to produce 2 moles of H 2O.
- Convert 4 moles of H 2to moles of H 2O using the mole ratio 2 H 2: 2 H 2O:
- Therefore, 4 moles of H 2will produce 4 moles of H 2O.
4 moles H 2× (2 moles H 2O / 2 moles H 2) = 4 moles H 2O
Limiting Reactants and Excess Reactants
Chemical reactions involve reactants and products. Sometimes, we have an excess of one reactant compared to the other. This leads to the concept of limiting reactants and excess reactants.
Limiting Reactant
The limiting reactant is the reactant that is completely consumed in a chemical reaction, limiting the amount of product that can be formed. It determines the maximum yield of the reaction.
Excess Reactant
The excess reactant is the reactant that is present in excess compared to the limiting reactant. It is not completely consumed in the reaction and remains after the reaction is complete.
Identifying the Limiting Reactant
To identify the limiting reactant, we compare the mole ratio of the reactants to their stoichiometric coefficients in the balanced chemical equation. The reactant with the smallest mole ratio is the limiting reactant.
Calculating Excess Reactant
Once the limiting reactant is identified, we can calculate the amount of excess reactant remaining using the stoichiometry of the reaction. The excess reactant is the difference between the initial amount and the amount consumed in the reaction.
Examples
Example 1:Reactants: 2 moles of A and 3 moles of BStoichiometry: A + 2B
> C
Mole ratio of A: 2/1 = 2Mole ratio of B: 3/2 = 1.5Since the mole ratio of B is smaller, B is the limiting reactant. Example 2:Reactants: 1 mole of A and 1.5 moles of BStoichiometry: 2A + B
> C
Mole ratio of A: 1/2 = 0.5Mole ratio of B: 1.5/1 = 1.5Since the mole ratio of A is smaller, A is the limiting reactant.
Percent Yield and Theoretical Yield
Chemical reactions rarely proceed to completion, meaning that not all of the reactants are converted into products. The percent yield is a measure of the efficiency of a chemical reaction and is defined as the ratio of the actual yield to the theoretical yield, multiplied by 100%.
The theoretical yield is the maximum amount of product that can be obtained from a given amount of reactants, assuming that the reaction proceeds to completion and there are no side reactions.
Calculating Percent Yield
The percent yield can be calculated using the following formula:
“`Percent yield = (Actual yield / Theoretical yield) x 100%“`
Where:
- Actual yield is the mass of product actually obtained from the reaction.
- Theoretical yield is the mass of product that would be obtained if the reaction proceeded to completion.
Factors Affecting Percent Yield, Chemistry mole packet answer key
Several factors can affect the percent yield of a reaction, including:
- Side reactions:Side reactions are competing reactions that consume reactants or produce unwanted products, reducing the yield of the desired product.
- Impurities:Impurities in the reactants can interfere with the reaction and reduce the yield.
- Reaction conditions:Factors such as temperature, pressure, and reaction time can affect the rate and extent of the reaction, influencing the yield.
- Catalyst:A catalyst is a substance that speeds up a reaction without being consumed. Catalysts can increase the percent yield by reducing the activation energy of the reaction.
- Experimental errors:Errors in measuring reactants, products, or reaction conditions can affect the accuracy of the percent yield calculation.
FAQ Corner
What is the purpose of the Chemistry Mole Packet?
The Chemistry Mole Packet provides comprehensive practice and reinforcement of key concepts related to mole calculations, stoichiometry, and chemical reactions.
How can I use the Chemistry Mole Packet Answer Key?
Refer to the answer key to check your solutions, identify areas for improvement, and reinforce your understanding of the concepts covered in the packet.
What are some common mistakes to avoid in mole calculations?
Pay close attention to units, ensure proper conversion factors, and double-check your calculations to minimize errors.